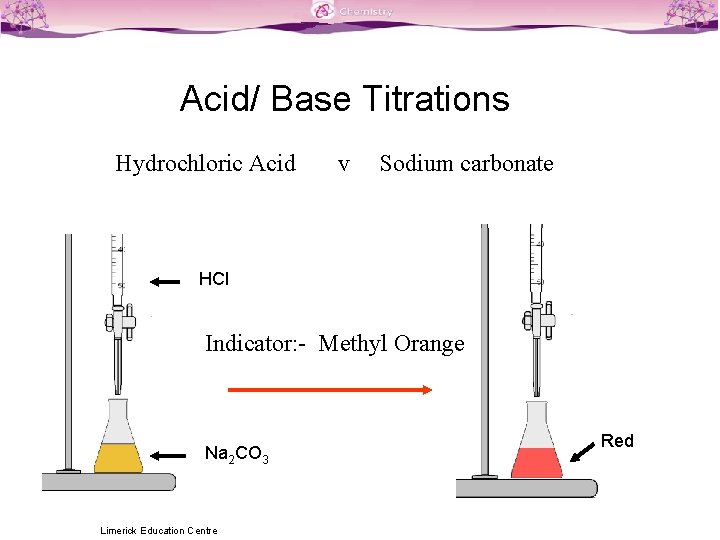
Titration Between Hcl And Naoh . both acid and base are strong, which not only makes determination of end point easy (steep part of the curve is. hcl + naoh → nacl + h 2 o. use this class practical to explore titration, producing the salt sodium chloride with sodium hydroxide and hydrochloric acid. hcl and naoh are strong acid and strong base respectively and their titration curves are similar (shape of curve) in different. In the neutralization of hydrochloric acid by sodium hydroxide, the mole ratio of acid to base is 1:1. Hydrochloric acid reacts with sodium hydroxide on the 1:1 basis. complete the following table and generate a titration curve showing the ph versus volume of added base for the titration of 50.0 ml.
from dxoxhgurz.blob.core.windows.net
complete the following table and generate a titration curve showing the ph versus volume of added base for the titration of 50.0 ml. both acid and base are strong, which not only makes determination of end point easy (steep part of the curve is. In the neutralization of hydrochloric acid by sodium hydroxide, the mole ratio of acid to base is 1:1. use this class practical to explore titration, producing the salt sodium chloride with sodium hydroxide and hydrochloric acid. hcl + naoh → nacl + h 2 o. hcl and naoh are strong acid and strong base respectively and their titration curves are similar (shape of curve) in different. Hydrochloric acid reacts with sodium hydroxide on the 1:1 basis.
Titration Of Naoh And Hcl Using Methyl Orange at Leonard Auger blog
Titration Between Hcl And Naoh hcl + naoh → nacl + h 2 o. hcl and naoh are strong acid and strong base respectively and their titration curves are similar (shape of curve) in different. hcl + naoh → nacl + h 2 o. use this class practical to explore titration, producing the salt sodium chloride with sodium hydroxide and hydrochloric acid. complete the following table and generate a titration curve showing the ph versus volume of added base for the titration of 50.0 ml. both acid and base are strong, which not only makes determination of end point easy (steep part of the curve is. In the neutralization of hydrochloric acid by sodium hydroxide, the mole ratio of acid to base is 1:1. Hydrochloric acid reacts with sodium hydroxide on the 1:1 basis.
From www.visionlearning.com
Acids and Bases I Chemistry Visionlearning Titration Between Hcl And Naoh hcl and naoh are strong acid and strong base respectively and their titration curves are similar (shape of curve) in different. use this class practical to explore titration, producing the salt sodium chloride with sodium hydroxide and hydrochloric acid. complete the following table and generate a titration curve showing the ph versus volume of added base for. Titration Between Hcl And Naoh.
From www.chegg.com
Solved Titration of HCl with standardized NaOH Titration Between Hcl And Naoh In the neutralization of hydrochloric acid by sodium hydroxide, the mole ratio of acid to base is 1:1. use this class practical to explore titration, producing the salt sodium chloride with sodium hydroxide and hydrochloric acid. hcl and naoh are strong acid and strong base respectively and their titration curves are similar (shape of curve) in different. Hydrochloric. Titration Between Hcl And Naoh.
From www.youtube.com
Titration of HCl with NaOH YouTube Titration Between Hcl And Naoh In the neutralization of hydrochloric acid by sodium hydroxide, the mole ratio of acid to base is 1:1. both acid and base are strong, which not only makes determination of end point easy (steep part of the curve is. complete the following table and generate a titration curve showing the ph versus volume of added base for the. Titration Between Hcl And Naoh.
From chemistrymadesimple.net
What is Titration and How is it Done? Chemistry Made Simple Titration Between Hcl And Naoh complete the following table and generate a titration curve showing the ph versus volume of added base for the titration of 50.0 ml. use this class practical to explore titration, producing the salt sodium chloride with sodium hydroxide and hydrochloric acid. Hydrochloric acid reacts with sodium hydroxide on the 1:1 basis. hcl + naoh → nacl +. Titration Between Hcl And Naoh.
From www.youtube.com
Titration of a monoprotic strong acid (HCl) and monoprotic strong base Titration Between Hcl And Naoh hcl and naoh are strong acid and strong base respectively and their titration curves are similar (shape of curve) in different. use this class practical to explore titration, producing the salt sodium chloride with sodium hydroxide and hydrochloric acid. complete the following table and generate a titration curve showing the ph versus volume of added base for. Titration Between Hcl And Naoh.
From www.slideserve.com
PPT Chemical Reactions PowerPoint Presentation, free download ID Titration Between Hcl And Naoh complete the following table and generate a titration curve showing the ph versus volume of added base for the titration of 50.0 ml. Hydrochloric acid reacts with sodium hydroxide on the 1:1 basis. both acid and base are strong, which not only makes determination of end point easy (steep part of the curve is. use this class. Titration Between Hcl And Naoh.
From www.youtube.com
Titration HCl and NaOH methyl orange YouTube Titration Between Hcl And Naoh hcl and naoh are strong acid and strong base respectively and their titration curves are similar (shape of curve) in different. In the neutralization of hydrochloric acid by sodium hydroxide, the mole ratio of acid to base is 1:1. Hydrochloric acid reacts with sodium hydroxide on the 1:1 basis. hcl + naoh → nacl + h 2 o.. Titration Between Hcl And Naoh.
From www.vrogue.co
The Graph Of Ph During The Titration Of Naoh And Hcl vrogue.co Titration Between Hcl And Naoh In the neutralization of hydrochloric acid by sodium hydroxide, the mole ratio of acid to base is 1:1. hcl + naoh → nacl + h 2 o. complete the following table and generate a titration curve showing the ph versus volume of added base for the titration of 50.0 ml. hcl and naoh are strong acid and. Titration Between Hcl And Naoh.
From studylib.net
3. Titration NaOH and HCl concentration of an alkali Titration Between Hcl And Naoh both acid and base are strong, which not only makes determination of end point easy (steep part of the curve is. use this class practical to explore titration, producing the salt sodium chloride with sodium hydroxide and hydrochloric acid. Hydrochloric acid reacts with sodium hydroxide on the 1:1 basis. hcl + naoh → nacl + h 2. Titration Between Hcl And Naoh.
From exotnsiwz.blob.core.windows.net
Titration Of Naoh With Hcl at Katherine Grassi blog Titration Between Hcl And Naoh both acid and base are strong, which not only makes determination of end point easy (steep part of the curve is. Hydrochloric acid reacts with sodium hydroxide on the 1:1 basis. hcl + naoh → nacl + h 2 o. In the neutralization of hydrochloric acid by sodium hydroxide, the mole ratio of acid to base is 1:1.. Titration Between Hcl And Naoh.
From dxoxhgurz.blob.core.windows.net
Titration Of Naoh And Hcl Using Methyl Orange at Leonard Auger blog Titration Between Hcl And Naoh complete the following table and generate a titration curve showing the ph versus volume of added base for the titration of 50.0 ml. In the neutralization of hydrochloric acid by sodium hydroxide, the mole ratio of acid to base is 1:1. both acid and base are strong, which not only makes determination of end point easy (steep part. Titration Between Hcl And Naoh.
From psiberg.com
The Equivalence Point Acid/Base Titrations PSIBERG Titration Between Hcl And Naoh hcl and naoh are strong acid and strong base respectively and their titration curves are similar (shape of curve) in different. both acid and base are strong, which not only makes determination of end point easy (steep part of the curve is. use this class practical to explore titration, producing the salt sodium chloride with sodium hydroxide. Titration Between Hcl And Naoh.
From studylib.net
Titration of HCl with NaOH Titration Between Hcl And Naoh In the neutralization of hydrochloric acid by sodium hydroxide, the mole ratio of acid to base is 1:1. hcl + naoh → nacl + h 2 o. Hydrochloric acid reacts with sodium hydroxide on the 1:1 basis. both acid and base are strong, which not only makes determination of end point easy (steep part of the curve is.. Titration Between Hcl And Naoh.
From www.youtube.com
Conductometric titration I strong acid (HCl) versus strong base Titration Between Hcl And Naoh hcl and naoh are strong acid and strong base respectively and their titration curves are similar (shape of curve) in different. Hydrochloric acid reacts with sodium hydroxide on the 1:1 basis. both acid and base are strong, which not only makes determination of end point easy (steep part of the curve is. hcl + naoh → nacl. Titration Between Hcl And Naoh.
From www.doubtnut.com
Which of the following plot represents the graph of pH against volume Titration Between Hcl And Naoh hcl + naoh → nacl + h 2 o. Hydrochloric acid reacts with sodium hydroxide on the 1:1 basis. In the neutralization of hydrochloric acid by sodium hydroxide, the mole ratio of acid to base is 1:1. both acid and base are strong, which not only makes determination of end point easy (steep part of the curve is.. Titration Between Hcl And Naoh.
From dxoqraial.blob.core.windows.net
Titration Reaction Of Hcl And Naoh at Lucia Chamberlain blog Titration Between Hcl And Naoh Hydrochloric acid reacts with sodium hydroxide on the 1:1 basis. hcl and naoh are strong acid and strong base respectively and their titration curves are similar (shape of curve) in different. hcl + naoh → nacl + h 2 o. both acid and base are strong, which not only makes determination of end point easy (steep part. Titration Between Hcl And Naoh.
From www.youtube.com
Titration of NaOH and HCl YouTube Titration Between Hcl And Naoh hcl + naoh → nacl + h 2 o. use this class practical to explore titration, producing the salt sodium chloride with sodium hydroxide and hydrochloric acid. hcl and naoh are strong acid and strong base respectively and their titration curves are similar (shape of curve) in different. Hydrochloric acid reacts with sodium hydroxide on the 1:1. Titration Between Hcl And Naoh.
From dxoqraial.blob.core.windows.net
Titration Reaction Of Hcl And Naoh at Lucia Chamberlain blog Titration Between Hcl And Naoh hcl and naoh are strong acid and strong base respectively and their titration curves are similar (shape of curve) in different. Hydrochloric acid reacts with sodium hydroxide on the 1:1 basis. hcl + naoh → nacl + h 2 o. complete the following table and generate a titration curve showing the ph versus volume of added base. Titration Between Hcl And Naoh.